 Loading... Please wait...
Loading... Please wait...- 970-759-1040 Text / Voice
- Gift Certificates
- My Account
Currency Displayed in
- Home
- Turquoise Learning Center
- Healing Powers of Turquoise
- The Physical Properties of Turquoise
Categories
The Physical Properties of Turquoise
The Physical Properties of Turquoise
Physical Properties of Turquoise & Turquoise Facts
The Physical Properties of Turquoise Or The Chemical Make-up Of Turquoise
Here are some brief facts on the Chemical make up of Turquoise: the physical properties of Turquoise are: hydrated phosphate of aluminum and copper (copper aluminum phosphate) or CuAl6((OH)2/PO4)4 CuAl6(PO4)4(OH)8 + 4H2O. In the language of chemists and geologists, turquoise is known as. Turquoise stones can contain impurities that form veins of sandstone, limonite, psilomelane or jasper. At temperatures of 500 degrees, blue Turquoise stones will become greener. Below are more Turquoise properties.
Physical Properties of Turquoise - Colors of Turquoise
 |
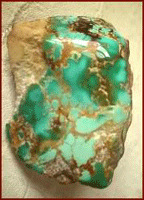
Turquoise comes in all shades of green and blue and in some rare instances lime green, yellow and even brown turquoise. The chemical makeup of Turquoise makes it a non-translucent stone whose most valuable specimens are a spider web Turquoise in almost any color, robin’s egg blue or deep-blue azure Turquoise in Europe, as well as some other rare looks such as Lime Green Turquoise, water web or birdseye matrix Turquoise, and pyrite matrix Turquoise. In American the physical properties of Turquoise all for spider web or pattern matrix bearing stones with great color which are the most valuable, most of which come from Arizona, Colorado, New Mexico, and Nevada. North American specimens also contain impurities that form matrix streaks within the stones. The veins are inclusions from nearby rock fragments or oxides and form during the creation of Turquoise. As mentioned above these veins can contain sandstones, limonite, malachite, chrysocolla jaspers or psilomelane. The veins in some stones interlock in patterns to form "spider-web" turquoise. One color the physical properties of Turquoise do not allow for in natural is pure or stark white Turquoise. |
The physical properties of Turquoise concerning the crystal habit of Turquoise
Nearly all Turquoise is cryptocrystalline. In fact, scientists had thought Turquoise was amorphous (non-crystal forming) until 1911, when crystalline specimens were found in Virginia.
The Turquoise crystal system is Triclinic, which is the least symmetrical of all crystal systems with all three axes of unequal lengths and none intersecting at right angles. The common physical properties of Turquoise or shape for this system is the pinacoid.
Turquoise Facts on the Geological EnvironmentThe common environment of Turquoise is arid or semiarid zones such as those that occur in Iran or the Desert Southwest of the United States. It is found in veins and nodules in weathered rhyolitic igneous rock or in Manganese and chert where it forms as a secondary mineral of the process known as hydrothermal replacement depositing that occurs when chemicals leach out of nearby rock by way of rain or a saturated water table. Copper eroding from deposits leaches into cracks and combines with phosphates and Turquoise’s other chemicals. These rare conditions contribute to the chemical makeup of Turquoise as well as the physical properties of Turquoise. |
 |
Chemical Enhancements on the Turquoise
Turquoise is a controversial stone because a lot of the stones sold have received so many chemical treatments that the final product is completely different from its original form. Enhancements can include, plastic, wax and oils that change color, durability and polish as well as heat treatment. The chemical makeup of Turquoise is such that it allows for enhancement and stabilization.
Physical Properties of Turquoise on Hardness
Turquoise has a hardness of 5.5 to 6.5 on Mohs Scale. The process by which Turquoise forms creates a porous physical properties of Turquoise in some stones. The harder, less porous stones polish better than the pale softer, chalky stones but these can have waxes or oils pressed into them to help their polish. Green Turquoise seems to be harder than the light blue Turquoise.
Imitators of Turquoise
Because of the rarity of fine specimens of Turquoise, jewelry makers have been creating imitations of it for centuries. The Egyptians used a glazed quartz paste as a substitute for the Turquoise for their jewelry requirements. The most common modern imitators of Turquoise are Howlite, Magnesite, Turquenite, dyed chalcedony, glasses, ceramics, and plastics. The minerals most often confused with Turquoise include Amazonite, Prosopite, Lazulite, Hemamorphite, Chrysocolla, Odontolite, Serpentine, Smithsonite, Faustite, and Variscite. Bone Turquoise or Vivianite (a hydrous ferrous phosphate) can leach into fossils and turn them a blue that is close to Turquoise in color.
Reconstituted Turquoise-The process of reconstituting Turquoise consists of pulverizing pieces of turquoise that are then stabilized and hardened with resins to achieve a natural Turquoise appearance. Resin-reconstituted Turquoise usually has an odor that allows for detection.
Lab-Grown Synthetic Turquoise: Also known as Neo-turquoise, Hamburger Turquoise or Neolite. Lab-grown Turquoise does not have the veins of impurities found in most American Turquoise. The refractive index of natural Turquoise is usually slightly higher than that of lab-grown stones. Genuine specimens also have homogenous blue matrices that contain irregular white particles.
Turquoise Care Facts
The most common dangers to Turquoise are scratches, sharp blows, hot water, and household chemicals. Because it is a hydrous stone, water or light can change the color of Turquoise stones and its relative softness can make it vulnerable to scratches. The pores of the stone will easily absorb body oils or other oils causing the stone to yellow over time. Do not use an ultrasonic cleaner on Turquoise and avoid chlorine.
Chem: CuAl6(PO4)4(OH)8 * 5H2O Hydrous copper aluminum phosphate
Color: sky blue, bluish-green, pale green, dark "forest" green, dark "sky" blue
Refrac. Index: 1.61 - 1.65
Birefraction: 0.04
Hardness: 5 - 6
Spec. Grav.: 2.60 - 2.80
Specific Gravity: 2.6 to 2.8
Cleavage: none
Fracture: conchoidal, uneven
Transparency: opaque
Chemical Makeup of Turquoise: CuAl6((OH)2/PO4)4 CuAl6(PO4)4(OH)8 + 4H2O
Luster: waxy, vitreous in macro-crystals
Color of streak: white, usually with brown or black spots, or white with greenish tint
Class: phosphates
Crystal System: triclinic; bar 1; rarely seen in crystalline form, most stones are cryptocrystalline
Best Field Indicators: crystal habit, hardness, luster, color and associations
See Also: TurquoiseFacts.com | Turquoise-Museum.com
Back to the Jewelry Learning Center
Thank you for reading about the Physical Properties of Turquoise and hopefully you have learned a little about the chemical makeup of Turquoise.












